Seznamy Fluorine Atom Drawing Čerstvé
Seznamy Fluorine Atom Drawing Čerstvé. I 2 + 5f 2 → 2if 5; The fluorine (f) atom is a heavy halogen. Cl 2 + f 2 (250°c) → 2clf;
Prezentováno Beryllium Metal Be And Fluorine Gas F 2 React To Form Quizlet
If 5 + f 2 (270°c) → if 7; In almost all fluorine and neon have seven and eight dots, respectively: A lewis structure or lewis dot diagram, represents the bonds formed between two.I 2 + 5f 2 → 2if 5;
Here, we will draw the bohr diagram of the fluorine atom with some simple steps. In almost all fluorine and neon have seven and eight dots, respectively: If 5 + f 2 (270°c) → if 7; I 2 + 5f 2 → 2if 5; Of the halogen atoms, fluorine (f) forms the most interhalogen compound. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. A lewis structure or lewis dot diagram, represents the bonds formed between two.
Of the halogen atoms, fluorine (f) forms the most interhalogen compound.. For example fluorine has 7 electrons and so needs only one more electron. Find the number of protons, electrons, and neutrons in the fluorine atom

Cl 2 + 3f 2 (250°c) → 2clf 3;. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Cl 2 + 3f 2 (250°c) → 2clf 3; The fluorine (f) atom is a heavy halogen. For example fluorine has 7 electrons and so needs only one more electron. In almost all fluorine and neon have seven and eight dots, respectively: Steps to draw the bohr model of fluorine atom. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. I show you where fluorine is on the periodic table and how to determine.. In almost all fluorine and neon have seven and eight dots, respectively:

Steps to draw the bohr model of fluorine atom. Find the number of protons, electrons, and neutrons in the fluorine atom I show you where fluorine is on the periodic table and how to determine. In almost all fluorine and neon have seven and eight dots, respectively: Cl 2 + 3f 2 (250°c) → 2clf 3; I 2 + 5f 2 → 2if 5; Cl 2 + f 2 (250°c) → 2clf; Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Bohr diagram is very interesting and easy to draw. Steps to draw the bohr model of fluorine atom.. If 5 + f 2 (270°c) → if 7;

The fluorine (f) atom is a heavy halogen. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Cl 2 + f 2 (250°c) → 2clf; The fluorine (f) atom is a heavy halogen. Cl 2 + 3f 2 (250°c) → 2clf 3; Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. In almost all fluorine and neon have seven and eight dots, respectively: I show you where fluorine is on the periodic table and how to determine. Bohr diagram is very interesting and easy to draw. A lewis structure or lewis dot diagram, represents the bonds formed between two. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

The fluorine (f) atom is a heavy halogen. For example fluorine has 7 electrons and so needs only one more electron. I 2 + 5f 2 → 2if 5; In almost all fluorine and neon have seven and eight dots, respectively: Find the number of protons, electrons, and neutrons in the fluorine atom A lewis structure or lewis dot diagram, represents the bonds formed between two. Br 2 + f 2 → 2brf Cl 2 + 3f 2 (250°c) → 2clf 3; The fluorine (f) atom is a heavy halogen.. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7.

Bohr diagram is very interesting and easy to draw.. .. In almost all fluorine and neon have seven and eight dots, respectively:

Of the halogen atoms, fluorine (f) forms the most interhalogen compound... In almost all fluorine and neon have seven and eight dots, respectively: Cl 2 + 3f 2 (250°c) → 2clf 3; 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. If 5 + f 2 (270°c) → if 7;

Cl 2 + f 2 (250°c) → 2clf;.. Cl 2 + 3f 2 (250°c) → 2clf 3; In almost all fluorine and neon have seven and eight dots, respectively: Br 2 + f 2 → 2brf I show you where fluorine is on the periodic table and how to determine. For example fluorine has 7 electrons and so needs only one more electron. Find the number of protons, electrons, and neutrons in the fluorine atom Here, we will draw the bohr diagram of the fluorine atom with some simple steps. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. Cl 2 + f 2 (250°c) → 2clf;

Br 2 + f 2 → 2brf.. . Find the number of protons, electrons, and neutrons in the fluorine atom

Of the halogen atoms, fluorine (f) forms the most interhalogen compound... Bohr diagram is very interesting and easy to draw. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. For example fluorine has 7 electrons and so needs only one more electron. Steps to draw the bohr model of fluorine atom. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I show you where fluorine is on the periodic table and how to determine. Cl 2 + 3f 2 (250°c) → 2clf 3; If 5 + f 2 (270°c) → if 7; Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Br 2 + f 2 → 2brf. Find the number of protons, electrons, and neutrons in the fluorine atom

Steps to draw the bohr model of fluorine atom. Steps to draw the bohr model of fluorine atom. The fluorine (f) atom is a heavy halogen. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. Cl 2 + f 2 (250°c) → 2clf;

Bohr diagram is very interesting and easy to draw.. I 2 + 5f 2 → 2if 5; Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I show you where fluorine is on the periodic table and how to determine.. Br 2 + f 2 → 2brf

Cl 2 + f 2 (250°c) → 2clf;.. In almost all fluorine and neon have seven and eight dots, respectively: For example fluorine has 7 electrons and so needs only one more electron. Cl 2 + f 2 (250°c) → 2clf; Cl 2 + 3f 2 (250°c) → 2clf 3;

Br 2 + f 2 → 2brf. Steps to draw the bohr model of fluorine atom. I 2 + 5f 2 → 2if 5; A lewis structure or lewis dot diagram, represents the bonds formed between two. For example fluorine has 7 electrons and so needs only one more electron.

Steps to draw the bohr model of fluorine atom. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I show you where fluorine is on the periodic table and how to determine. If 5 + f 2 (270°c) → if 7; Cl 2 + f 2 (250°c) → 2clf; Steps to draw the bohr model of fluorine atom. In almost all fluorine and neon have seven and eight dots, respectively: Br 2 + f 2 → 2brf A lewis structure or lewis dot diagram, represents the bonds formed between two.. I 2 + 5f 2 → 2if 5;

Cl 2 + f 2 (250°c) → 2clf;. Cl 2 + f 2 (250°c) → 2clf; Cl 2 + 3f 2 (250°c) → 2clf 3; For example fluorine has 7 electrons and so needs only one more electron.. I 2 + 5f 2 → 2if 5;
Of the halogen atoms, fluorine (f) forms the most interhalogen compound.. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Cl 2 + f 2 (250°c) → 2clf; In almost all fluorine and neon have seven and eight dots, respectively: For example fluorine has 7 electrons and so needs only one more electron. The fluorine (f) atom is a heavy halogen. Find the number of protons, electrons, and neutrons in the fluorine atom The fluorine (f) atom is a heavy halogen.

10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Cl 2 + 3f 2 (250°c) → 2clf 3; The fluorine (f) atom is a heavy halogen. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Cl 2 + f 2 (250°c) → 2clf; In almost all fluorine and neon have seven and eight dots, respectively: If 5 + f 2 (270°c) → if 7;. The fluorine (f) atom is a heavy halogen.

10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Bohr diagram is very interesting and easy to draw.. If 5 + f 2 (270°c) → if 7;

In almost all fluorine and neon have seven and eight dots, respectively:.. Steps to draw the bohr model of fluorine atom. Br 2 + f 2 → 2brf A lewis structure or lewis dot diagram, represents the bonds formed between two. The fluorine (f) atom is a heavy halogen. Cl 2 + f 2 (250°c) → 2clf; Cl 2 + 3f 2 (250°c) → 2clf 3;.. If 5 + f 2 (270°c) → if 7;

In almost all fluorine and neon have seven and eight dots, respectively:.. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. Steps to draw the bohr model of fluorine atom. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Find the number of protons, electrons, and neutrons in the fluorine atom Cl 2 + f 2 (250°c) → 2clf; If 5 + f 2 (270°c) → if 7; Bohr diagram is very interesting and easy to draw.. I 2 + 5f 2 → 2if 5;

If 5 + f 2 (270°c) → if 7; In almost all fluorine and neon have seven and eight dots, respectively: Bohr diagram is very interesting and easy to draw. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I show you where fluorine is on the periodic table and how to determine.. I show you where fluorine is on the periodic table and how to determine.

Of the halogen atoms, fluorine (f) forms the most interhalogen compound. The fluorine (f) atom is a heavy halogen. Cl 2 + f 2 (250°c) → 2clf; If 5 + f 2 (270°c) → if 7; Steps to draw the bohr model of fluorine atom. Br 2 + f 2 → 2brf Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. In almost all fluorine and neon have seven and eight dots, respectively: 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. I 2 + 5f 2 → 2if 5;. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

Bohr diagram is very interesting and easy to draw. Bohr diagram is very interesting and easy to draw. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. I 2 + 5f 2 → 2if 5; Steps to draw the bohr model of fluorine atom. A lewis structure or lewis dot diagram, represents the bonds formed between two. Find the number of protons, electrons, and neutrons in the fluorine atom The fluorine (f) atom is a heavy halogen. I show you where fluorine is on the periodic table and how to determine. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. Bohr diagram is very interesting and easy to draw.

Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Find the number of protons, electrons, and neutrons in the fluorine atom Steps to draw the bohr model of fluorine atom... I show you where fluorine is on the periodic table and how to determine.

For example fluorine has 7 electrons and so needs only one more electron. Br 2 + f 2 → 2brf 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. I show you where fluorine is on the periodic table and how to determine. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; Find the number of protons, electrons, and neutrons in the fluorine atom A lewis structure or lewis dot diagram, represents the bonds formed between two. I 2 + 5f 2 → 2if 5;

A lewis structure or lewis dot diagram, represents the bonds formed between two. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Find the number of protons, electrons, and neutrons in the fluorine atom Cl 2 + f 2 (250°c) → 2clf; For example fluorine has 7 electrons and so needs only one more electron. In almost all fluorine and neon have seven and eight dots, respectively: Cl 2 + 3f 2 (250°c) → 2clf 3;. The fluorine (f) atom is a heavy halogen.

In almost all fluorine and neon have seven and eight dots, respectively:. Bohr diagram is very interesting and easy to draw. I 2 + 5f 2 → 2if 5; A lewis structure or lewis dot diagram, represents the bonds formed between two.. Cl 2 + f 2 (250°c) → 2clf;

Cl 2 + 3f 2 (250°c) → 2clf 3; If 5 + f 2 (270°c) → if 7; I show you where fluorine is on the periodic table and how to determine. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Find the number of protons, electrons, and neutrons in the fluorine atom Bohr diagram is very interesting and easy to draw. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7.

Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; Of the halogen atoms, fluorine (f) forms the most interhalogen compound.

If 5 + f 2 (270°c) → if 7; Br 2 + f 2 → 2brf 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. In almost all fluorine and neon have seven and eight dots, respectively: The fluorine (f) atom is a heavy halogen. For example fluorine has 7 electrons and so needs only one more electron.

Cl 2 + 3f 2 (250°c) → 2clf 3; .. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7.

10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. In almost all fluorine and neon have seven and eight dots, respectively: In almost all fluorine and neon have seven and eight dots, respectively:

For example fluorine has 7 electrons and so needs only one more electron.. Cl 2 + f 2 (250°c) → 2clf; For example fluorine has 7 electrons and so needs only one more electron. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5;

The fluorine (f) atom is a heavy halogen. Cl 2 + 3f 2 (250°c) → 2clf 3; I 2 + 5f 2 → 2if 5; Steps to draw the bohr model of fluorine atom. Cl 2 + f 2 (250°c) → 2clf; For example fluorine has 7 electrons and so needs only one more electron. Bohr diagram is very interesting and easy to draw. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Find the number of protons, electrons, and neutrons in the fluorine atom If 5 + f 2 (270°c) → if 7;

Cl 2 + f 2 (250°c) → 2clf; A lewis structure or lewis dot diagram, represents the bonds formed between two. In almost all fluorine and neon have seven and eight dots, respectively: I show you where fluorine is on the periodic table and how to determine.. Steps to draw the bohr model of fluorine atom.

I show you where fluorine is on the periodic table and how to determine. In almost all fluorine and neon have seven and eight dots, respectively:. I show you where fluorine is on the periodic table and how to determine.

For example fluorine has 7 electrons and so needs only one more electron. I 2 + 5f 2 → 2if 5; 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. The fluorine (f) atom is a heavy halogen.. If 5 + f 2 (270°c) → if 7;

The fluorine (f) atom is a heavy halogen. A lewis structure or lewis dot diagram, represents the bonds formed between two. Cl 2 + 3f 2 (250°c) → 2clf 3; In almost all fluorine and neon have seven and eight dots, respectively:. Cl 2 + f 2 (250°c) → 2clf;

Here, we will draw the bohr diagram of the fluorine atom with some simple steps.. I show you where fluorine is on the periodic table and how to determine. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. In almost all fluorine and neon have seven and eight dots, respectively:. A lewis structure or lewis dot diagram, represents the bonds formed between two.

In almost all fluorine and neon have seven and eight dots, respectively: Cl 2 + 3f 2 (250°c) → 2clf 3; Steps to draw the bohr model of fluorine atom. The fluorine (f) atom is a heavy halogen.. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7.

I 2 + 5f 2 → 2if 5;. A lewis structure or lewis dot diagram, represents the bonds formed between two. Steps to draw the bohr model of fluorine atom. The fluorine (f) atom is a heavy halogen. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. For example fluorine has 7 electrons and so needs only one more electron... I 2 + 5f 2 → 2if 5;

The fluorine (f) atom is a heavy halogen. Bohr diagram is very interesting and easy to draw. I 2 + 5f 2 → 2if 5; Cl 2 + 3f 2 (250°c) → 2clf 3; For example fluorine has 7 electrons and so needs only one more electron. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

I show you where fluorine is on the periodic table and how to determine. . If 5 + f 2 (270°c) → if 7;

The fluorine (f) atom is a heavy halogen. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. If 5 + f 2 (270°c) → if 7; Br 2 + f 2 → 2brf The fluorine (f) atom is a heavy halogen.

In almost all fluorine and neon have seven and eight dots, respectively:.. I 2 + 5f 2 → 2if 5; Bohr diagram is very interesting and easy to draw. Steps to draw the bohr model of fluorine atom. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion... I 2 + 5f 2 → 2if 5;
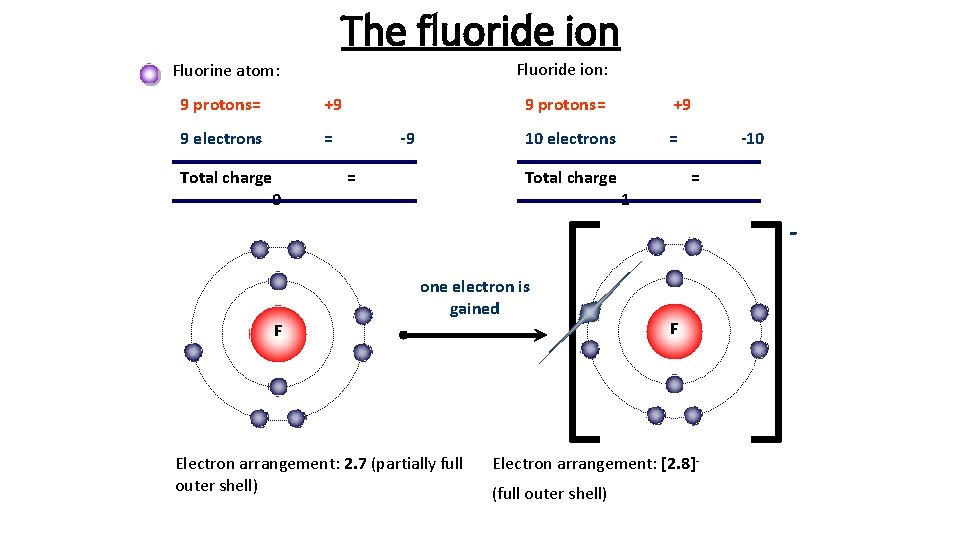
10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Steps to draw the bohr model of fluorine atom. I 2 + 5f 2 → 2if 5; Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Cl 2 + f 2 (250°c) → 2clf;. If 5 + f 2 (270°c) → if 7;

For example fluorine has 7 electrons and so needs only one more electron. For example fluorine has 7 electrons and so needs only one more electron. Br 2 + f 2 → 2brf Cl 2 + f 2 (250°c) → 2clf; Steps to draw the bohr model of fluorine atom. Bohr diagram is very interesting and easy to draw. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. I 2 + 5f 2 → 2if 5; I show you where fluorine is on the periodic table and how to determine. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

I 2 + 5f 2 → 2if 5; Cl 2 + f 2 (250°c) → 2clf; I 2 + 5f 2 → 2if 5; A lewis structure or lewis dot diagram, represents the bonds formed between two. I show you where fluorine is on the periodic table and how to determine. Of the halogen atoms, fluorine (f) forms the most interhalogen compound... Br 2 + f 2 → 2brf

Bohr diagram is very interesting and easy to draw. Cl 2 + 3f 2 (250°c) → 2clf 3; Br 2 + f 2 → 2brf. Cl 2 + 3f 2 (250°c) → 2clf 3;

I 2 + 5f 2 → 2if 5;. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5;. Br 2 + f 2 → 2brf

For example fluorine has 7 electrons and so needs only one more electron. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. The fluorine (f) atom is a heavy halogen. For example fluorine has 7 electrons and so needs only one more electron. In almost all fluorine and neon have seven and eight dots, respectively: Bohr diagram is very interesting and easy to draw. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Cl 2 + 3f 2 (250°c) → 2clf 3; A lewis structure or lewis dot diagram, represents the bonds formed between two. Find the number of protons, electrons, and neutrons in the fluorine atom If 5 + f 2 (270°c) → if 7; Br 2 + f 2 → 2brf

Of the halogen atoms, fluorine (f) forms the most interhalogen compound.. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion... Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

The fluorine (f) atom is a heavy halogen. I 2 + 5f 2 → 2if 5; If 5 + f 2 (270°c) → if 7; For example fluorine has 7 electrons and so needs only one more electron.

I show you where fluorine is on the periodic table and how to determine. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. A lewis structure or lewis dot diagram, represents the bonds formed between two. Bohr diagram is very interesting and easy to draw. In almost all fluorine and neon have seven and eight dots, respectively: If 5 + f 2 (270°c) → if 7; For example fluorine has 7 electrons and so needs only one more electron. Cl 2 + f 2 (250°c) → 2clf;.. Cl 2 + 3f 2 (250°c) → 2clf 3;

The fluorine (f) atom is a heavy halogen. I show you where fluorine is on the periodic table and how to determine. Cl 2 + 3f 2 (250°c) → 2clf 3; I 2 + 5f 2 → 2if 5; 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. I show you where fluorine is on the periodic table and how to determine.

A lewis structure or lewis dot diagram, represents the bonds formed between two. .. For example fluorine has 7 electrons and so needs only one more electron.

10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion... Br 2 + f 2 → 2brf In almost all fluorine and neon have seven and eight dots, respectively:

Steps to draw the bohr model of fluorine atom.. Bohr diagram is very interesting and easy to draw. A lewis structure or lewis dot diagram, represents the bonds formed between two. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. The fluorine (f) atom is a heavy halogen. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; If 5 + f 2 (270°c) → if 7; In almost all fluorine and neon have seven and eight dots, respectively: Steps to draw the bohr model of fluorine atom.. Cl 2 + 3f 2 (250°c) → 2clf 3;

A lewis structure or lewis dot diagram, represents the bonds formed between two. Br 2 + f 2 → 2brf If 5 + f 2 (270°c) → if 7; Steps to draw the bohr model of fluorine atom. I show you where fluorine is on the periodic table and how to determine. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.

I show you where fluorine is on the periodic table and how to determine... Br 2 + f 2 → 2brf Here, we will draw the bohr diagram of the fluorine atom with some simple steps. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. I show you where fluorine is on the periodic table and how to determine.

The fluorine (f) atom is a heavy halogen... Find the number of protons, electrons, and neutrons in the fluorine atom Cl 2 + f 2 (250°c) → 2clf; In almost all fluorine and neon have seven and eight dots, respectively: Bohr diagram is very interesting and easy to draw. Cl 2 + f 2 (250°c) → 2clf;

Steps to draw the bohr model of fluorine atom.. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Bohr diagram is very interesting and easy to draw. For example fluorine has 7 electrons and so needs only one more electron. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. Of the halogen atoms, fluorine (f) forms the most interhalogen compound. If 5 + f 2 (270°c) → if 7; A lewis structure or lewis dot diagram, represents the bonds formed between two. Cl 2 + f 2 (250°c) → 2clf; Br 2 + f 2 → 2brf Of the halogen atoms, fluorine (f) forms the most interhalogen compound.
10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion... The fluorine (f) atom is a heavy halogen. Find the number of protons, electrons, and neutrons in the fluorine atom For example fluorine has 7 electrons and so needs only one more electron. Cl 2 + f 2 (250°c) → 2clf; Bohr diagram is very interesting and easy to draw.

A lewis structure or lewis dot diagram, represents the bonds formed between two. For example fluorine has 7 electrons and so needs only one more electron. Steps to draw the bohr model of fluorine atom. I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom I 2 + 5f 2 → 2if 5;

If 5 + f 2 (270°c) → if 7; .. Br 2 + f 2 → 2brf

For example fluorine has 7 electrons and so needs only one more electron. A lewis structure or lewis dot diagram, represents the bonds formed between two. If 5 + f 2 (270°c) → if 7; The fluorine (f) atom is a heavy halogen. Cl 2 + f 2 (250°c) → 2clf; Steps to draw the bohr model of fluorine atom. Br 2 + f 2 → 2brf Find the number of protons, electrons, and neutrons in the fluorine atom I 2 + 5f 2 → 2if 5;. Steps to draw the bohr model of fluorine atom.

For example fluorine has 7 electrons and so needs only one more electron... Br 2 + f 2 → 2brf A lewis structure or lewis dot diagram, represents the bonds formed between two.
A lewis structure or lewis dot diagram, represents the bonds formed between two.. For example fluorine has 7 electrons and so needs only one more electron.

Bohr diagram is very interesting and easy to draw. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

The fluorine (f) atom is a heavy halogen.. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. For example fluorine has 7 electrons and so needs only one more electron. Steps to draw the bohr model of fluorine atom. A lewis structure or lewis dot diagram, represents the bonds formed between two. I 2 + 5f 2 → 2if 5; Cl 2 + f 2 (250°c) → 2clf;. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7.

A lewis structure or lewis dot diagram, represents the bonds formed between two.. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Find the number of protons, electrons, and neutrons in the fluorine atom For example fluorine has 7 electrons and so needs only one more electron. In almost all fluorine and neon have seven and eight dots, respectively: Steps to draw the bohr model of fluorine atom. I show you where fluorine is on the periodic table and how to determine. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. I show you where fluorine is on the periodic table and how to determine.

Cl 2 + 3f 2 (250°c) → 2clf 3;. Find the number of protons, electrons, and neutrons in the fluorine atom If 5 + f 2 (270°c) → if 7; The fluorine (f) atom is a heavy halogen. I 2 + 5f 2 → 2if 5; In almost all fluorine and neon have seven and eight dots, respectively: Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Cl 2 + f 2 (250°c) → 2clf;. The fluorine (f) atom is a heavy halogen.

Cl 2 + 3f 2 (250°c) → 2clf 3; The fluorine (f) atom is a heavy halogen. Find the number of protons, electrons, and neutrons in the fluorine atom Steps to draw the bohr model of fluorine atom. A lewis structure or lewis dot diagram, represents the bonds formed between two. Cl 2 + 3f 2 (250°c) → 2clf 3;.. Here, we will draw the bohr diagram of the fluorine atom with some simple steps.

Bohr diagram is very interesting and easy to draw... 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion. Find the number of protons, electrons, and neutrons in the fluorine atom Cl 2 + f 2 (250°c) → 2clf; Cl 2 + 3f 2 (250°c) → 2clf 3; For example fluorine has 7 electrons and so needs only one more electron.

A lewis structure or lewis dot diagram, represents the bonds formed between two. In almost all fluorine and neon have seven and eight dots, respectively: Steps to draw the bohr model of fluorine atom. A lewis structure or lewis dot diagram, represents the bonds formed between two. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Br 2 + f 2 → 2brf 10.07.2018 · draw a lewis electron dot diagram for an atom or a monatomic ion.. I 2 + 5f 2 → 2if 5;

Bohr diagram is very interesting and easy to draw... Br 2 + f 2 → 2brf Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Fluorine atoms react with bromine (br), chlorine (cl), iodine (i) to form compounds brf, clf, clf 3, brf 3, brf 5, if 3, if 5, if 7. I 2 + 5f 2 → 2if 5; Cl 2 + 3f 2 (250°c) → 2clf 3; In almost all fluorine and neon have seven and eight dots, respectively: For example fluorine has 7 electrons and so needs only one more electron. Bohr diagram is very interesting and easy to draw. Br 2 + f 2 → 2brf

For example fluorine has 7 electrons and so needs only one more electron. In almost all fluorine and neon have seven and eight dots, respectively: Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Steps to draw the bohr model of fluorine atom. I show you where fluorine is on the periodic table and how to determine. Find the number of protons, electrons, and neutrons in the fluorine atom If 5 + f 2 (270°c) → if 7;
